Chapter 1 Introduction
In modern industrial production, the surface treatment and recycling of metal materials has become a crucial component of both the manufacturing and recycling sectors. Among these processes, tin and silver stripping are particularly prominent. These two processes are not only relevant to the electronics, information technology, jewelry, and photography industries, but are also closely linked to the recovery and reuse of precious metals.
Tin and silver, as common metals, are widely used in welding, plating, protective coatings, electroplating, and electroforming processes. Tin, with its excellent solderability and corrosion resistance, plays a crucial role in soldering for printed circuit boards (PCBs) and electronic components. Silver, due to its excellent electrical and thermal conductivity, is widely used in the surface treatment of electronic components, electrical contacts, circuits, as well as jewelry, utensils, and imaging materials.
However, with product rework, the accumulation of waste, and the emergence of defective products during the production process, large amounts of tin and silver waste are inevitably generated. If this waste is not properly handled, it not only wastes precious metals but also causes serious environmental pollution. In particular, direct discharge of silver-containing waste fixer fluid and tin-containing electronic scrap can cause heavy metal pollution in water bodies, posing a threat to the ecological environment and human health.
Against this backdrop, the research and application of tin and silver stripping processes have emerged. Through scientifically sound chemical, electrochemical, or physically assisted methods, the tin or silver layer on the substrate is effectively stripped away. The tin or silver layer is then recovered and purified, allowing it to be reintegrated into the production cycle. This not only achieves resource reuse but also aligns with the principles of green production and a circular economy.
The tin and silver stripping process has a wide range of applications:
——In electronic scrap recycling, it’s used to remove the solder layer, facilitating further sorting and metal recovery;
——In the jewelry and handicraft manufacturing industry, it’s used to rework defective products to avoid total scrap;
——In the photography and imaging industry, it’s used to recover silver from fixer and film, improving precious metal utilization;
——In the electroplating and electroforming industries, it’s used to remove substandard plating or electroforming layers to ensure product quality.
Therefore, systematically reviewing the principles of tin and silver stripping, including specific processes, equipment selection, environmental protection measures, and application cases, will not only help enhance industry awareness of resource recycling but also provide companies with practical reference solutions for their operations.
Chapter 2 Basic Principles
Although the tin stripping and silver stripping processes differ in application scenarios and specific processes, they both follow the basic principle of metal layer stripping: through chemical or electrochemical reactions, the metal layer is converted into soluble ions or complexes, thereby separating it from the substrate.
2.1 Chemical principles
(1)Chemical dissolution of tin
A common method of tin stripping is to use a solution containing a strong oxidant or chelating agent to oxidize metallic tin into divalent tin ions (Sn²⁺), thereby removing it from the substrate surface.
Common reactions include:![]() (In a strong acid environment, tin is oxidized to stannous chloride and dissolves.)
(In a strong acid environment, tin is oxidized to stannous chloride and dissolves.)
Another situation is with the help of strong oxidizing agents such as nitric acid:![]()
(During this process, the tin is completely oxidized and forms a stannous nitrate solution.)
(2)Chemical dissolution of silver
Silver does not react easily with dilute acid, but is easily oxidized in nitric acid:
![]()
In cyanide-containing solutions, silver forms a stable complex with cyanide ions:
![]()
Therefore, early silver removal processes widely used cyanide systems, but due to their severe toxicity and environmental risks, they are now gradually being replaced by thiosulfate, ammonia complexes or environmentally friendly organic ligands.
2.2Electrochemical principles
In addition to relying on chemical agents, electrolysis is also commonly used for tin and silver stripping:
——In an electrolytic cell, the workpiece to be stripped serves as the anode. An applied current causes metallic tin or silver to enter the solution as ions.
——Simultaneously, hydrogen or other reduction products are released at the cathode.
——Current density, electrolyte composition, and temperature are key parameters determining efficiency and quality.
For example:
Electrolytic stripping reaction of silver in cyanide solution:
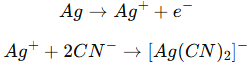
Electrolytic stripping of tin:
![]()
This method has the advantages of high speed and high controllability and is suitable for large-scale industrial operations.
2.3 Physical and auxiliary methods
In addition to purely chemical or electrochemical methods, there are also some auxiliary methods:
-Mechanical assistance: Sandblasting and ultrasonic vibrations help separate the coating from the substrate.
-Heat treatment: High temperatures are used to promote the thermal expansion difference between the metal layer and the substrate, accelerating delamination.
-Environmentally friendly alternatives: New organic chelating agents or biological extracts are also being explored in the desilvering process to reduce hazardous waste.
These methods are often combined with chemical or electrochemical processes to improve efficiency and environmental friendliness.
Chapter 3 Application Scenarios
The detinning and desilvering process has a wide range of applications, ranging from electronic waste recycling, jewelry manufacturing rework, and imaging material recycling to quality control in electroplating and electroforming production lines. The following sections analyze its main application areas.
3.1 Electronic waste disposal
Electronic waste (E-waste) is one of the fastest-growing sources of solid waste globally. With the rapid evolution of smartphones, computers, and home appliances, millions of tons of discarded electronics are generated annually. E-waste often contains significant amounts of solder and silver plating:
-Printed circuit boards (PCBs): Surfaces are covered with tin solder joints and small amounts of silver-plated wires;
-Connectors and plugs: Silver plating is often used to reduce contact resistance;
-Chips and components: Some packages utilize silver-containing conductive adhesives or solder.
For example, in one ton of waste circuit boards, solder accounts for about 30-40kg and silver contains about 200-300g. If they are effectively recycled through the tin and silver stripping process, resource utilization and economic benefits can be greatly improved.
3.2 Jewelry Manufacturing and Rework
Gold and silver jewelry manufacturing often requires electroplating or electroforming to enhance the product’s appearance and wear resistance. However, due to improper operation or customer returns, a certain percentage of defective and reworked products are produced.
-For silver or tin-plated jewelry, simply discarding it not only wastes precious metals but also increases costs for the manufacturer.
-Desilvering allows the silver layer to be removed and re-electroplated or reprocessed.
-For gold and silver electroformed parts, desilvering can be used to remove the outer layer, and then the silver can be recovered through precious metal recovery equipment.
3.3 Photographic Imaging Industry
The fixer and film emulsion used in traditional photography contain large amounts of silver salts. With the widespread adoption of digital imaging, many photofinishing companies have closed down, but a significant amount of waste fixer still needs to be disposed of.
-One liter of waste fixer typically contains 2–5 grams of silver. Directly discharging this waste not only pollutes water sources but also wastes resources.
-Through the desilvering process, the silver ions can be reduced and deposited into silver powder or ingots.
-This process complies with environmental regulations and generates economic benefits.
Therefore, many environmental protection and recycling companies have prioritized the recycling of waste fixer.
3.4 Electroplating and Electroforming Industry
During the electroplating and electroforming production process, products often require stripping:
-If the plating layer is uneven, has pinholes, or has discoloration, it needs to be stripped and reprocessed;
-When reworking electroformed jewelry samples or failed products, silver or gold stripping is required.
Chapter 4 Detailed Process Flow
Although the process flow of tin and silver stripping varies depending on the application field, it can be roughly divided into the following stages: pretreatment → stripping reaction → separation and recovery → waste liquid treatment → refining and reuse.
4.1 Pre-treatment Stage
Before de-tinning and de-silvering, the workpieces or scrap materials require pre-treatment to ensure a smooth process:
a.Cleaning: Removes surface oil, dust, and flux residue;
b.Sorting: Separates workpieces of different materials and coatings to avoid cross-contamination;
c.Mechanical Assistance: Crushing, shearing, or grinding is performed as necessary to increase contact area and improve reaction efficiency.
4.2 Stripping reaction stage
This is the core step of the entire process. Depending on the target, chemical, electrochemical or physical assisted methods can be selected.
-Commonly used reagents: Nitric acid, sodium thiosulfate, and cyanide substitutes.
-Electrolysis: Silver ions are introduced into an electrolyte solution under low voltage and a steady current.
-Stripping: Silver ions are converted to Ag⁺ or complexes.
4.3 Separation and recovery
The solution after tin and silver stripping contains a large amount of metal ions, which need to be recovered by precipitation, electrolysis or adsorption:
-Tin recovery: hydrolysis precipitation is often used to convert Sn²⁺ into SnO₂ precipitate;
-Silver recovery: can be achieved through chlorination (forming AgCl), electrolysis (precipitating silver powder), adsorption resin, etc.
4.4 Wastewater treatment
The tin and silver stripping process inevitably produces waste liquid, which will cause serious pollution if not treated. Common treatment methods include:
a.Neutralization: Adjust waste acid and alkali to neutrality.
b.Precipitation: Use lime and sulfide to precipitate heavy metals.
c.Membrane separation: Use reverse osmosis or ion exchange membranes to separate metal ions.
d.Reuse: Some wastewater can be recycled, reducing treatment costs.
4.5 Refining and Recycling
Finally, the recovered tin or silver needs to be further purified for industrial or commercial use:
-Tin: can be smelted into metal ingots for use in solder or alloy production;
-Silver: can be electrolytically purified to 999 or 9999 purity and used in jewelry, electrical appliances or investment products.
Through this series of processes, the tin and silver stripping process not only achieves the recovery of precious metals, but also ensures the sustainability of production.
Chapter 5: Cost Analysis of Tin and Silver Stripping Process (with Fuzzy Data Version)
The cost of the tin and silver stripping process consists of five parts: raw materials and chemicals, equipment investment, energy consumption, labor costs and environmental protection treatment.
1.Raw Material and Chemical Costs
Common tin and silver stripping powder consumption is approximately 1–3 kg per ton of scrap (depending on the tin/silver ratio of the scrap). The market price is roughly US$15–40 per kg (with significant variations between suppliers). For example, for one ton of standard electronic scrap, the chemical cost is approximately US$20–100.
2.Equipment Investment
-Small laboratory equipment: approximately $3,000–8,000 per unit.
-Medium-sized industrial equipment: approximately $10,000–25,000 per unit.
-Large, fully automated production lines: can cost upwards of $50,000.
Depreciation costs, calculated over the equipment’s useful life (typically 5–8 years), are typically less than $10 per ton of waste.
3.Energy and labor
-Power Consumption: Typical electrolysis equipment uses 5–15 kW of power. Based on a 6–8 hour batch operation, the electricity cost per ton of waste is approximately $5–15 USD (depending on local electricity prices).
-Labor Cost: In Asia, this typically ranges from $20–50 USD per person per day, while in Europe and the United States, this can exceed $100 USD per day. The typical labor cost per ton of waste is 1–2 people per day.
4.Waste Liquid Treatment and Environmental Protection Costs
-The waste liquid contains residual chemicals and heavy metal ions and needs to be treated.
-The cost of third-party environmental treatment is generally between US$30 and US$80 per ton (with large differences between different countries).
Chapter 6: Market Prospects and Profit Space (with Fuzzy Data Version)
Tin and silver stripping is not only an environmentally friendly process, but also a commercially viable recycling method. Profit margins depend primarily on precious metal content, market prices, and process efficiency.
1.Profit Source
Precious metal value:
-The typical recycling rate for scrap tin is 85–95%, while that for scrap silver can reach 90–98%.
-Current market prices (2025): $20–30/kg for tin and $600–800/kg for silver.
-If a ton of scrap contains 200–500 grams of silver, its market value is approximately $120–400; if it contains 1–5 kilograms of tin, it can generate a profit of $20–150.
2.Factors affecting profits
-Variation in raw material metal content: Different batches of scrap contain varying proportions of tin and silver, resulting in significant variations in recycling value.
-Process efficiency: High-efficiency equipment can achieve a 10–15% higher recovery rate than less efficient processes, directly increasing profits.-
-Market volatility: Silver and tin prices fluctuate, potentially fluctuating by ±20% in the short term, significantly impacting profits.
-Scale: When processing scrap in bulk, unit costs decrease, increasing profit margins.





